Mass Number of an atom is the total number of protons and neutrons present in an atom. We know that an atom consists of electrons, protons, and neutrons but the mass of the atom is contributed by protons and neutrons as the mass of electrons is very low hence it doesn't contribute to the mass of an atom. In this article, we will learn what is the mass number, the mass number definition, the mass number formula, and the mass number of some commonly known elements.
What is Atomic Number?
Atomic number of an element is the total number of protons that are present in the atom. The atomic number of an atom explains the properties of an element, it is represented using the letter "Z". All the elements are arranged in the periodic table on the basis of their atomic number.
For example,
- Atomic number of oxygen is 8
- Atomic number of carbon is 6
We represent the atomic number as,
- 7Z (Atomic Number of Nitrogen)
What is Mass Number?
Mass Number of an atom also called Atomic Mass Number is the total number of protons and neutrons present in an atom. Rutherford in his gold foil experiment concluded that the mass of the atom is concentrated in a small region called the Nucleus which is positively charged. Later on, it was found that the nucleus consists of two particles Protons and Neutrons. Neutrons and Protons are together called nucleons. Proton is a positively charged subatomic particle while Neutron is a neutral particle. The mass of Proton and Neutron is the same and is equal to 1.67 ⨯10-27 kg. Compared to Proton and Neutron, an electron is 1000 times lighter as the mass of an electron is 9.1 ⨯ 10-31 kg
Mass Number Definition
Mass Number is defined as the sum of the total number of protons and neutrons present in the nucleus of atoms. It gives the idea of how heavier is the atom of an element.
Mass Number Formula
Mass Number is represented using the letter 'A' and we know that it is the total number of protons and atoms in an atom. Hence, the Mass Number Formula is given as:
Mass Number (A) = Number of Protons + Number of Neutrons
Mass Number Example
Some examples of the Mass Number are mentioned below:
Example 1: Hydrogen Atom has 1 proton, and no neutron hence the mass number of hydrogen is 1.
Example 2: Helium has 2 Protons and 2 Neutrons, hence, the mass number of Helium is 4.
Example 3: Sodium has 11 Protons and 12 Neutrons, hence the mass number of sodium is 23.
Mass Number Properties
The Properties of Mass Number are mentioned below:
- Mass Number is sum total of the numbers of protons and neutrons present in an atom.
- The symbol of Mass Number is A.
- Mass Number for an Element can be different for different atoms of the same element. These are called isotopes. Example C-12, C-13 and C-14
- Mass Numbers for two elements can be the same for atoms of two different elements. Such a pair of atoms are called isobars. Examples include Ar-40 and Ca-40 both have the same mass number but different atomic numbers as they are different elements.
How to Find Mass Number?
Mass Number of an atom is the total number of protons and neutrons present in the mass number. Mass Number can be calculated using the following steps:
Step 1: First look for the Atomic Number of the element as the Atomic Number is equal to the mass number.
Step 2: Now look for the number of neutrons in the atom.
Step 3: Add the Number of Protons i.e. Atomic Number and the number of neutrons to get the Mass Number of the element.
Calculation of Number of Neutrons
The formula used for calculating Mass Number can be modified to calculate the number of protons. We know that mass number is the sum of the number of protons i.e. Atomic Number and the Number of Neutrons. Hence, the Number of Neutrons can be calculated by subtracting Atomic Number from Mass Number.
Number of Neutrons = Mass Number - Number of Protons
OR
Number of Neutrons = Mass Number - Atomic Number
Mass Number of Elements
As we have already learned that Mass Number of an Element is the sum total of the number of protons and neutrons. Mass Number of elements is denoted by the letter 'A'. Let's learn the mass number of some elements.
Mass Number of Hydrogen
Hydrogen is the very first element in the modern periodic table. Hydrogen is the most abundant element found in the universe. Hydrogen is denoted by the letter 'H'. The atomic number of Hydrogen is 1. It means it has one proton. But it has no neutrons. Hence, the mass number of hydrogen is 1.
Mass Number of Lithium
Lithium is the first element of the second period in the modern periodic table. The Atomic Number of Lithium is 3. The number of neutrons present in Lithium is 4. Hence, the Mass Number of Lithium is 7.
Mass Number of Carbon
Carbon is one of the most important in the periodic table. It is the main element found in any organic compound. The atomic carbon of Carbon is 6, hence the number of protons in carbon is 6. The number of neutrons in carbon is 6. Hence the mass number of Carbon is 12.
Mass Number of Nitrogen
Nitrogen is the most abundant gas found in the atmosphere. Nitrogen is denoted by the letter 'N'. The atomic number of Nitrogen is 7. Hence, the number of protons in Nitrogen is 7. The number of neutrons in Nitrogen is also 7. Thus, the Mass Number of Nitrogen is 14.
Mass Number of Oxygen
Oxygen is an important non-metallic element. Oxygen supports life and combustion. Oxygen is denoted by the letter 'O'. The atomic number of oxygen is 8. Hence, it has 8 protons. Oxygen has also 8 neutrons, hence the mass number of Oxygen is 16.
List of Mass Numbers of First 20 Elements
The mass number and the atomic number of different elements of the periodic table are as follows,
Element | Symbol | Atomic Number (Z) | Mass Number (A) | Number of Protons | Number of Electrons | Number of Neutrons (A-Z) |
|---|
Hydrogen | H | 1 | 1 | 1 | 1 | 0 |
|---|
Helium | He | 2 | 4 | 2 | 2 | 2 |
|---|
Lithium | Li | 3 | 7 | 3 | 3 | 4 |
|---|
Beryllium | Be | 4 | 9 | 4 | 4 | 5 |
|---|
Boron | B | 5 | 11 | 5 | 5 | 6 |
|---|
Carbon | C | 6 | 12 | 6 | 6 | 6 |
|---|
Nitrogen | N | 7 | 14 | 7 | 7 | 7 |
|---|
Oxygen | O | 8 | 16 | 8 | 8 | 8 |
|---|
Fluorine | F | 9 | 19 | 9 | 9 | 10 |
|---|
Neon | Ne | 10 | 20 | 10 | 10 | 10 |
|---|
Sodium | Na | 11 | 23 | 11 | 11 | 12 |
|---|
Magnesium | Mg | 12 | 24 | 12 | 12 | 12 |
|---|
Aluminum | Al | 13 | 27 | 13 | 13 | 14 |
|---|
Silicon | Si | 14 | 28 | 14 | 14 | 14 |
|---|
Phosphorus | P | 15 | 31 | 15 | 15 | 16 |
|---|
Sulfur | S | 16 | 32 | 16 | 16 | 16 |
|---|
Chlorine | Cl | 17 | 35 | 17 | 17 | 18 |
|---|
Argon | Ar | 18 | 40 | 18 | 18 | 22 |
|---|
Potassium | K | 19 | 39 | 19 | 19 | 20 |
|---|
Calcium | Ca | 20 | 40 | 20 | 20 | 20 |
|---|
Atomic Number and Mass Number
Atomic Number is the number of protons in an atom while Mass Number is the total number of protons and neutrons in an atom. Atomic Number is denoted by the letter 'Z' and the Mass Number is denoted by the letter 'A'. Atomic Number and Mass Number of an element are different however this is not the case always. In the case of Hydrogen, the Atomic Number and Mass Number both are equal to 1.
In general, the mass number is generally larger than the Atomic Number as the mass number takes account into both numbers of protons and the number of neutrons. Thus Atomic Number differs from the Mass Number by the number of neutrons present in the atom.
Difference between Mass Number(A) and Atomic Number(Z)
The difference between valency, A, and Z is discussed below:
- The electrons present in the outermost shell of an atom are known as the valence electrons and their combining capacity to react and form molecules with other atoms of the same or different elements is known as the valency of the atom.
- The valence shell of the atom is the last shell in which electrons fill. It can accommodate 8 electrons and after that, it became chemically inactive and its valency becomes zero.
- Thus the valency of an atom is defined as the number of atoms shared by the valance shell of the electron to achieve its octet state.
- Mass number (A) is the number of nucleons i.e. protons and neutrons that any nucleus of the atom has.
- Atomic number (Z) is the number of protons that any atom has.
Representation of an Atom
The atom of any element is represented by using the English Alphabet and its notation is discussed in the image below,
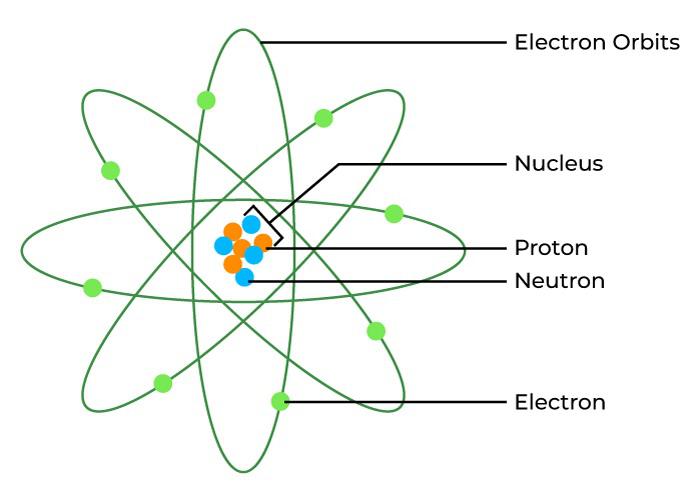
Atomic Mass vs Mass Number
Atomic Number and Mass Number both indicate the mass of an atom but they have some differences between them. Let's learn the difference between Atomic Mass and Mass Number through the following table:
Atomic Number | Mass Number |
|---|
It is the weighted average of the mass of an atom in the natural stage which also includes its isotopes | It is the total number of protons and neutron present in an atom |
Atomic Mass takes into account all the isotopes that exist | Mass number is calculated differently for different isotopes |
Atomic Mass can be fractional or in decimal | Mass Number is always a whole number |
Unit of Atomic Mass is atomic mass unit. | Mass Number has no units |
Atomic Mass is the same for all the atoms of an element regardless | Mass Number is different for different isotopes |
Also, Check,
Solved Examples on Mass Number
Example 1: Find the number of electrons, protons, and neutrons of an atom with Atomic Number (Z) 13 and Mass Number (A) 26.
Solution:
Given,
Z = 13 and A = 26
- Atomic Number = Number of Protons = Z =13
- Number of Electrons = Number of Protons = 13
- Mass Number = Number of Neutrons = A - Z = 26 - 13 = 13
Example 2: Find the number of electrons, protons, and neutrons of an atom with atomic number(Z) 19 and mass number(A) 39.
Solution:
For the atom given above,
Number of Protons = Z
= 19
Number of Electrons = Number of Protons
= Z
= 19
Number of Neutrons = A - Z
= 39 - 19
= 20
Similar Reads
Chapter 1 Some Basic Concepts of Chemistry
Importance of Chemistry in Everyday LifeImportance of Chemistry in Everyday Life: The scientific study of matter's properties and behavior is known as chemistry. It is a natural science that studies the elements that makeup matter, as well as the compounds, made up of atoms, molecules, and ions: their composition, structure, qualities, an
10 min read
Molecular Nature of Matter - Definition, States, Types, ExamplesThe distinct forms that different phases of matter take on is called the state of matter. The most common state matter that is easily observable in daily life is - Solid, liquid, gas and plasma. There are many other states known to us like - Bose-Einstein condensate and neutron degenerate matter, bu
9 min read
Properties of MatterEvery matter has its own set of properties. Physical and chemical properties can be used to classify these properties. Physical properties are those that may be measured or observed without affecting the substance's identity or composition. Physical properties include odor, color, density, and so on
9 min read
System of UnitsMeasurement forms the fundamental principle to various other branches of science, that is, construction and engineering services. Measurement is defined as the action of associating numerical with their possible physical quantities and phenomena. Measurements find a role in everyday activities to a
9 min read
Mass and WeightMass and Weight are commonly used in the same manner by the general masses but there are differences between both Mass and Weight, where Mass is the measure of Inertia unlike Weight which is a measure of force acting on a body towards the heavy body. But yet still many people use these two terms int
10 min read
Significant FiguresIn order to find the value of different sizes and compare them, measurement is used. Measuring things is not only a concept but also practically used in everyday life, for example, a milkman measures milk before selling it in order to make sure the correct amount is served, A tailor always measures
7 min read
Laws of Chemical CombinationLaws of Chemical Combination are one of the most fundamental building blocks of the subject of chemistry. As in our surrounding different matter reacts with each other and form various kind of different substances. Laws of Chemical Combination are the collection of laws that explains how these subst
7 min read
Law of Conservation of MassLaw of Conservation of Mass: The law of conservation of mass states that the mass can neither be created nor destroyed in a chemical reaction. This implies, in a closed system the mass of the elements involved initially in a chemical reaction is equal to the mass of the product obtained by the react
9 min read
Laws of Chemical CombinationLaws of Chemical Combination are one of the most fundamental building blocks of the subject of chemistry. As in our surrounding different matter reacts with each other and form various kind of different substances. Laws of Chemical Combination are the collection of laws that explains how these subst
7 min read
Gay Lussac's LawGay Lussac's is one of the Ideal gas laws that relates the pressure of the gas with its absolute temperature when its volume is kept constant. The basic statement of Gay Lussac’s Law is, the pressure produced by a gas is directly proportional to its temperature if mass and volume are kept fixed. Gay
6 min read
Dalton's Atomic TheoryIn the year 1808, the English scientist and chemist John Dalton proposed Dalton's atomic hypothesis, a scientific theory on the nature of matter. It asserted that all matter is made up of atoms, which are tiny, indivisible units. According to Dalton's atomic theory, all substances are made up of ato
8 min read
Atomic MassAtomic mass is the total mass of all subatomic particles of an atom, including protons, neutrons, and electrons. However, the mass of electrons is incredibly small, so it's typically neglected when determining an atom's overall mass. The unit commonly used to express atomic mass is the Atomic Mass U
9 min read
Molecular MassMolecular Mass is the mass of all the atoms present in a molecule. In ancient India and Greece, philosophers have first given the idea of atoms and deeply studied them. Around 500 BC.Everything around is made up of very small units these units are atoms in the language of science, very small in the
8 min read
Formula Mass of Ionic CompoundsThere are many known compounds and molecules. The compounds made up only of ions are called ionic compounds. The concept of the formula unit of ionic compounds and the formula mass helps find the atomic masses of the ionic compounds. The formula mass is then calculated in the same way in which the m
6 min read
Percentage Composition - Definition, Formula, ExamplesDifferent constituent elements make up any chemical compound. In some chemical reaction calculations, you'll need to figure out how much of a certain element is in a specific compound. Or, in order to understand the contribution of a specific element in any of the stoichiometric calculations of a ch
5 min read
Stoichiometry and Stoichiometric CalculationsJeremias Richter, a German chemist, was the first to create or discover the word Stoichiometry. The quantitative analysis of the reactants and products involved in a chemical reaction is known as chemical stoichiometry. The name "stoichiometry" comes from the Greek words "stoikhein" (element) and "m
7 min read
Chapter 2 Structure of Atom
Discovery of ElectronsThe basic idea of the discovery of the elementary particles was generated by Dalton's Atomic Theory. John Dalton in 1808 gave the first scientific theory about atoms, in which, he stated that atoms are the smallest particle of any matter. They are indivisible and indestructible. According to Dalton
7 min read
What is a Proton?Protons are the fundamental particles that reside inside the nucleus of any atom. They are the positive charge particle and are responsible for balancing the negative charge of the electron to make the atom electrically neutral. Proton was discovered by the famous scientist Ernest Rutherford. Atoms
6 min read
NeutronsNeutrons are fundamental subatomic particles of the atom. An atom is made up of electrons, protons and neutrons. James Chadwick, an English physicist, discovered the neutron in 1932. Neutrons are particles with no charge and higher mass. They are represented by n. They reside inside the nucleus of t
8 min read
Thomson's Atomic ModelThomson's Atomic Model is one of the fundamental models of the atom that tries to explain the working and structure of the atom. this model was proposed by famous Scientist JJ Thomson in 1904. Thomson during his cathode ray experiment proved the existence of a negatively charged particle called elec
6 min read
Rutherford Atomic ModelRutherford Atomic Model was proposed by Ernest Rutherford in 1911. It is also called the Planetary Model of the Atom. It introduced the concept of a dense, positively charged nucleus at the center of an atom, with electrons orbiting around it, forming the basis for modern atomic theory. In this arti
6 min read
Mass NumberMass Number of an atom is the total number of protons and neutrons present in an atom. We know that an atom consists of electrons, protons, and neutrons but the mass of the atom is contributed by protons and neutrons as the mass of electrons is very low hence it doesn't contribute to the mass of an
11 min read
Bohr's Model of an AtomBohr's Model is an atomic model proposed by Danish Physicist Niels Bohr in 1913. According to this model, in an atom, the electrons revolve around the nucleus in definite energy levels called orbits/shells. This model provides a basic understanding of the concept of the atom and its constituents. Le
8 min read
Planck's Quantum FormulaMaxwell's proposal concerning the wave nature of electromagnetic radiation was useful in describing phenomena such as interference, diffraction, and other phenomena as science progressed. However, he was unable to explain a number of other observations, including the nature of radiation emission fro
7 min read
Atomic SpectraAtomic Spectra is the spectrum of radiation of electromagnetic waves produced due to the transition of an electron from one energy level to another level within an atom. Atoms have an equal number of negative and positive charges. Atoms were described as spherical clouds of positive charges with emb
9 min read
Spectrum of the Hydrogen AtomElectrons in a hydrogen atom circle around a nucleus. Because of the electromagnetic force between the proton and electron, electrons go through numerous quantum states. Neil Bohr's model helps in visualizing these quantum states as electrons orbit the nucleus in different directions. When Electrons
7 min read
Bohr's Model of the Hydrogen AtomThe Bohr model of the hydrogen atom was the first atomic model to successfully explain the atomic hydrogen radiation spectra. Niels Bohr proposed the atomic Hydrogen model in 1913. The Bohr Model of the Hydrogen Atom attempts to fill in some of the gaps left by Rutherford's model. It has a special p
9 min read
Quantum Mechanical Atomic ModelSchrödinger used the electron's wave-particle duality to design and solve a difficult mathematical equation that precisely represented the behaviour of the electron in a hydrogen atom in 1926. The solution to Schrödinger's equation yielded the quantum mechanical model of the atom. The quantization o
8 min read
Quantum NumbersQuantum numbers in Chemistry, are the sets of numbers that describe an electron's orbit and movement within an atom. When the quantum numbers of all the electrons in a given atom are added together, they must satisfy the Schrodinger equation. Quantum numbers are the set of numbers used to describe t
12 min read
Electronic Configuration in Periods and GroupsElectronic Configuration is the arrangement of electrons in orbitals around an atomic nucleus. Electronic Configuration of a molecule refers to the distribution of electrons in various molecular orbitals. The number of electrons in bonding and antibonding molecular orbitals of a molecule or molecula
9 min read
Chapter 3 Classification of Elements and Periodicity in Properties
Dobereiners Triads - Definition, Types, LimitationsSince ancient times, there have been various attempts to classify the elements into groups according to their properties. As the new elements were discovered, a number of theories came up to classify the elements. Various scientists used various approaches and facts to justify their classification.
5 min read
Newland’s Law of OctavesNewland's Law of Octaves also called Law of Octaves was one of the initial attempts to arrange all the known chemical elements in a table to make their study better. In Newland's Law of Octaves, elements are arranged in the increasing order of their atomic mass and it is seen that the property of th
6 min read
Modern Periodic LawAll matter in our environment is made up of basic units known as elements. Initially, only 31 chemical elements were discovered in 1800 and it was easier to study their chemical and other properties. However, as more and more elements were discovered due to technological advancements in science, it
6 min read
Nomenclature of Elements with Atomic Number above 100The contemporary periodic table has around 118 elements. In most cases, the element's discoverer is given the honour of naming the element. The chemical element's name is derived from its physical or chemical properties, its origin, or mythical qualities. The IUPAC then approves the preferred name o
5 min read
Electron ConfigurationElectron Configuration of an element tells us how electrons are filled inside various orbitals of the atom. The distribution of electrons inside various orbital of atoms is very useful in explaining various properties of the atoms and their combination with other atoms. The electron configuration of
8 min read
p-Block Elements - Definition, Properties, Uses, ExamplesSome metals, all nonmetals, and metalloids are among these elements. Normal or representative elements are s-block and p-block elements combined (except zero group elements). Each periodic table period concludes with a member of the zero group (18th group), i.e. a noble gas with a closed shell ns2np
7 min read
Electronic Configuration of the d-block ElementsElectronic Configuration of the d-block elements are those that can be found in the contemporary periodic table from the third to the twelfth groups. These elements' valence electrons are located in the d orbital. d-block elements are sometimes known as transition elements or transition metals. The
7 min read
Chapter 4 Chemical Bonding and Molecular Structure
Ionic BondIonic Bond is a bond that is formed by the electrostatic force of attraction between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond formation. This bond is formed by the attracting force between the cations and the anions that are formed by the donating
8 min read
Bond Parameters - Definition, Order, Angle, LengthSeveral bond parameters, such as bond length, bond angle, bond order, and bond energy, can be used to characterize covalent bonds (also known as bond enthalpy). These bond parameters provide information about the stability of a chemical compound as well as the strength of the chemical bonds that hol
7 min read
VSEPR TheoryVSEPR Theory tells us about the basic structure of the molecules based on the force of repulsion between lone pair and bond pair of electrons. It states that any molecule arranged in such a structure minimizes the repulsion between the lone pair and bond pair of the molecule. Let's learn more about
9 min read
Valence Bond TheoryValence bond theory (VBT) describes the formation of covalent bonds and the electronic structure of molecules. It assumes that electrons occupy atomic orbitals of individual atoms within a molecule, and that the electrons of one atom are attracted to the nucleus of another atom. VBT states that the
7 min read
HybridizationThe concept of hybridization is defined as the process of combining two atomic orbitals to create a new type of hybridized orbitals. This intermixing typically results in the formation of hybrid orbitals with completely different energies, shapes, and so on. Hybridization is primarily carried out by
7 min read
Molecular Orbital TheoryThe Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as resonance-stabil
7 min read
Hydrogen BondingIn chemistry, a hydrogen bond is an electrostatic force of attraction between a hydrogen atom and another electronegative atom. It is a special type of dipole-dipole force. Hydrogen bonding is the phenomenon of the formation of Hydrogen Bonds. H Bonds are stronger than any dipole-dipole bonds but we
13 min read
Chapter 5 States of Matter
Intermolecular Forces - Definition, Types, Equations, ExamplesCharacteristics of chemical systems are observable when they represent the bulk properties of matter. For example, an individual molecule does not boil, while a bulk boils. Collections of water molecules have wetting properties while individual molecules do not. Water, just like all matter, can exis
8 min read
Intermolecular Forces - Definition, Types, Equations, ExamplesCharacteristics of chemical systems are observable when they represent the bulk properties of matter. For example, an individual molecule does not boil, while a bulk boils. Collections of water molecules have wetting properties while individual molecules do not. Water, just like all matter, can exis
8 min read
Intermolecular Forces - Definition, Types, Equations, ExamplesCharacteristics of chemical systems are observable when they represent the bulk properties of matter. For example, an individual molecule does not boil, while a bulk boils. Collections of water molecules have wetting properties while individual molecules do not. Water, just like all matter, can exis
8 min read
Intermolecular Forces - Definition, Types, Equations, ExamplesCharacteristics of chemical systems are observable when they represent the bulk properties of matter. For example, an individual molecule does not boil, while a bulk boils. Collections of water molecules have wetting properties while individual molecules do not. Water, just like all matter, can exis
8 min read
Gas LawsGas Laws, When the conditions are normal, all gases have similar behaviour. However, even slight changes in physical conditions such as pressure, temperature, or volume cause a deviation. The behaviour of gases is studied using gas laws. A gas's state variables, such as pressure, volume, and tempera
10 min read
Gas LawsGas Laws, When the conditions are normal, all gases have similar behaviour. However, even slight changes in physical conditions such as pressure, temperature, or volume cause a deviation. The behaviour of gases is studied using gas laws. A gas's state variables, such as pressure, volume, and tempera
10 min read
Gas LawsGas Laws, When the conditions are normal, all gases have similar behaviour. However, even slight changes in physical conditions such as pressure, temperature, or volume cause a deviation. The behaviour of gases is studied using gas laws. A gas's state variables, such as pressure, volume, and tempera
10 min read
Gas LawsGas Laws, When the conditions are normal, all gases have similar behaviour. However, even slight changes in physical conditions such as pressure, temperature, or volume cause a deviation. The behaviour of gases is studied using gas laws. A gas's state variables, such as pressure, volume, and tempera
10 min read
Gas LawsGas Laws, When the conditions are normal, all gases have similar behaviour. However, even slight changes in physical conditions such as pressure, temperature, or volume cause a deviation. The behaviour of gases is studied using gas laws. A gas's state variables, such as pressure, volume, and tempera
10 min read
Ideal Gas LawThe ideal gas law also called the general gas equation, is an equation that provides the relation among the various parameters of the gas i.e. they provide the relation among pressure(P), temperature(T), and Volume(V) of the gas. It is a combination of Charles’s law, Boyle’s Law, Avogadro’s law, and
10 min read
Derivation of Ideal Gas EquationThe ideal gas law is a well-defined approximation of the behaviour of several gases under various situations in thermodynamics. The Ideal Gas Equation is a mathematical formula that uses a combination of empirical and physical constants to express the states of hypothetical gases. The general gas eq
9 min read
Kinetic Energy and Molecular SpeedsTo study the action of molecules scientists have thought to study a theoretical model and that model is the Kinetic theory of gases and it assumes that molecules are very small relative to the distance between molecules. Typically, the actual properties of solids and fluids can be depicted by their
6 min read
Kinetic Molecular Theory of GasesThe kinetic molecular theory of gases explains a gas's three macroscopic characteristics in terms of the microscopic nature of the gas's atoms and molecules. The size, shape, mass, and volume of solids and liquids are commonly used to characterize their physical properties. Gases, on the other hand,
9 min read
Deviation of Real Gases from Ideal Gas BehaviourA state of matter is one of the different forms. In everyday life, four states of matter are visible: solid, liquid, gas, and plasma. Many intermediate states, such as liquid crystal, are known to exist, and certain states, such as Bose-Einstein condensates, neutron-degenerate matter, and quark-gluo
9 min read
Liquefaction of GasesPhysics and chemistry are both concerned with the study of matter, energy, and their interactions. Scientists know that matter can change states and that the sum of a system's matter and energy is constant because of thermodynamic rules. Matter changes state when energy is added or removed, forming
8 min read
Chapter 6 Thermodynamics
Basics Concepts of ThermodynamicsThermodynamics is concerned with the ideas of heat and temperature, as well as the exchange of heat and other forms of energy. The branch of science that is known as thermodynamics is related to the study of various kinds of energy and its interconversion. The behaviour of these quantities is govern
12 min read
Enthalpy Change of a ReactionThe study of thermodynamics is the study of systems that are too large to be extrapolated by mechanics alone. For many generations, thermodynamics was vaguely understood, and many of the results were determined only experimentally. Some of the results posed great theoretical challenges for physicist
9 min read
Enthalpies for Different Types of ReactionsThermodynamics is a field of physics that studies the relationship between heat, work, and temperature, as well as their relationships with energy, entropy, and the physical properties of matter and radiation. The four principles of thermodynamics regulate the behaviour of these quantities, which pr
10 min read
What is Spontaneity? - Definition, Types, Gibbs EnergyThermodynamics is a discipline of physics that studies heat, work, and temperature, as well as their relationships with energy, radiation, and matter's physical characteristics. The four principles of thermodynamics regulate the behaviour of these quantities, which provide a quantitative description
7 min read
Gibbs Energy Change and EquilibriumEnergy can take many forms, including kinetic energy produced by an object's movement, potential energy produced by an object's position, heat energy transferred from one object to another due to a temperature difference, radiant energy associated with sunlight, the electrical energy produced in gal
10 min read
Chapter 7 Equilibrium
Equilibrium in Physical ProcessesEquilibrium exists in physical processes, just as it does in chemical reactions. The equilibrium that arises between different states or phases of a substance, such as solid, liquid, and gas, is referred to as this. Let's take a closer look at how equilibrium works in physical processes. Substances
11 min read
Equilibrium in Chemical ProcessesChemical equilibrium is the state of a system in which the reactant and product concentrations do not change over time and the system's attributes do not change further. Reactions take place in both forward and reverse directions. When the rates of the forward and reverse reactions are similar in su
7 min read
Law of Chemical Equilibrium and Equilibrium ConstantDuring a chemical process, chemical equilibrium refers to the state in which the concentrations of both reactants and products have no tendency to fluctuate over time. When the forward and reverse reaction rates are equal, a chemical reaction is said to be in chemical equilibrium. The state is known
8 min read
Applications of Equilibrium ConstantsWhen a chemical process reaches equilibrium, the equilibrium constant (usually represented by the symbol K) provides information on the relationship between the products and reactants. For example, the equilibrium constant of concentration (denoted by Kc) of a chemical reaction at equilibrium can be
6 min read
What is the Relation between Equilibrium Constant, Reaction Quotient and Gibbs Energy?A scientist was observing a reaction and at a certain point and found the concentration of reactant is equal to the concentration of product and after some time and observed color of reactant is changing, the scientist found concentration of products is greater than the concentration of reactants, f
8 min read
Ionic EquilibriumReactants and products coexist in equilibrium, therefore reactant conversion to product is never greater than 100%. Equilibrium reactions may entail the breakdown of a covalent (non-polar) reactant or the ionisation of ionic compounds in polar solvents into their ions. This part will teach us about
5 min read
Acids, Bases and SaltsAcids, Bases, and Salts are the main chemical compounds that exist in our surroundings. Acids, Bases, and Salts are compounds that occur naturally and can also be created artificially. They are found in various substances including our food. Vinegar or acetic acid is used as a food preservative. Cit
15+ min read
Ionization of Acids and BasesIonization of a compound in Chemistry is the process by which neutral molecules are divided into charged ions in a solution. According to the Arrhenius Theory, acids are substances that dissociate in an aqueous medium to produce hydrogen ions, H+ ions, and bases are substances that dissociate in an
6 min read
Importance of pH in Everyday LifeAcids, bases, and salts have an impact on chemistry as well as our daily lives. Acids have a sour flavour (the word acid comes from the Latin word ‘acere’ which means ‘sour’), bases have a bitter taste, while salts themselves have a salty taste. Citric acid is found in fruits such as oranges and lem
13 min read
Strength of AcidsAcids are a molecule or other species which can donate a proton or accept an electron pair in reactions. When acids react with H2O, they create hydrogen ions; the strength of an acid is determined by the concentration of hydrogen ions in a solution. A higher number of hydrogen ions indicates that th
6 min read
Buffer SolutionBuffer Solution is a special aqueous solution that resists the change in its pH when some quantity of acid and Base is added. Many fluids, such as blood, have specific pH values of 7.14, and variations in these values indicate that the body is malfunctioning. The change in pH of Buffer Solutions on
10 min read
Solubility EquilibriaThe word "solubility product" refers to inexpensively soluble salts. It is the greatest product of the molar concentration of the ions (raised to their appropriate powers) produced by compound dissociation. The solubility product is constant at any given temperature. The lower the solubility product
5 min read
Chapter 8 Redox Reactions
Chapter 9 Hydrogen
Dihydrogen - Structure, Properties and ApplicationsThe lightest element is hydrogen. Under normal conditions, hydrogen is a gas composed of diatomic molecules with the formula H2. It is colourless, odourless, non-toxic, and extremely flammable. Hydrogen is the most abundant chemical element in the universe, accounting for roughly 75% of all normal m
7 min read
Isotopes of HydrogenIsotopes of an atom are variants of the same atom but with different mass numbers. That is if two atoms have the same atomic number but different mass numbers then they are called the isotopes of one another. Various examples of the isotopes are the isotopes of hydrogen, we have three different isot
10 min read
HydridesHydride in Chemistry is the name of a compound containing Hydrogen Anion. Hydrides are chemical compounds with one atom of hydrogen and an extra atom. Hence, they are an anion of Hydrogen. An anion is a species that has extra electrons and thus exhibits a negative charge. Thus, hydride (hydrogen ani
6 min read
Structure and Properties of WaterWater is a valuable natural resource. Water is essential for the survival of all living things. We can't imagine a world without water. Water is required by animals and plants to complete their daily metabolic activities. Water is required by plants to synthesize their food through the photosynthesi
9 min read
Chemical Formula of Water - Structure, Properties, Uses, Sample QuestionsHydrogen (H) has unique kind properties that are not like some other component on our planet, close to 66% of our Universe's mass is made out of this unique component. It is both electropositive as well as electronegative, as it structures hydrogen particles (H+)as well as hydride particles (H-). Hy
4 min read
Dihydrogen as Fuel - Definition, Uses, ExamplesHydrogen is the first element on the modern periodic table. It has the simplest atomic structure as compared to all other elements. In atomic form, it has one proton and one electron. On the other hand, in elemental form, it exists as a diatomic (H2) molecule called dihydrogen. If Hydrogen loses its
7 min read
Chapter 10 S-block Elements
Alkali MetalsAlkali metals are the first group of s-block elements that are found on the leftmost side of the periodic table. Alkali metals are the most electropositive elements on the periodic table as they easily lose electrons. These metals formed various useful compounds with halides, oxygen, and sulfur. Alk
11 min read
Characteristics of the Compounds of Alkali MetalsThere are a large number of elements around us having different properties and different uses based on those properties. For using these elements, properties are important so there should be some table to group these elements. This table is known as a periodic table which is created by using the wor
6 min read
Anomalous Behavior of Lithium and BerylliumElements are arranged in a periodic table row-wise and column-wise according to similarities in their chemical and physical properties. The elements in the first column are known as Group 1 elements which have the following elements lithium, sodium, potassium, rubidium, Caesium and Francium. All the
8 min read
Some Important Compounds of SodiumSodium is a soft metal, it is the eleventh element in the periodic table. It is represented by the Na symbol and the atomic number of sodium is 11 it belongs to the family of s-block elements in the periodic table. Sodium is the sixth most abundant element. Its amount in the earth's crust is nearly
7 min read
What is Sodium Chloride? - Definition, Preparation, Properties, UsesSalt's chemical name is sodium chloride. Sodium is an electrolyte that regulates your body's water content. Sodium is also involved in nerve impulses and muscle contractions. Sodium chloride is a medication used to treat or prevent sodium loss caused by dehydration, excessive sweating, or other fact
6 min read
Alkaline Earth MetalsAlkaline Earth Metals are Group 2 elements which includes a collection of elements Beryllium, Magnesium, Calcium, Barium, Strontium, and Radium, which are soft silver metals with a less metallic quality than Group 1 alkali metals. All the heavier metals in Group II such as Ca, Sr, Ba, and Ra, share
11 min read
Characteristics of the Compounds of Alkaline Earth MetalsAll the elements that exist in nature are arranged in a periodic table after several years of research work, these are placed in groups and rows based on some predefined criteria. Some elements may not follow the criteria but still, they are placed in the same column or group due to their similariti
8 min read
Anomalous Behavior of Lithium and BerylliumElements are arranged in a periodic table row-wise and column-wise according to similarities in their chemical and physical properties. The elements in the first column are known as Group 1 elements which have the following elements lithium, sodium, potassium, rubidium, Caesium and Francium. All the
8 min read
Some Important Compounds of CalciumCalcium is a reactive alkaline earth metal that when exposed to the air generates a black oxide-nitride coating. Its physical and chemical properties are most similar to those of strontium and barium, its heavier homologues. After iron and aluminium, it is the fifth most abundant element in the Eart
7 min read
Plaster of ParisPlaster of Paris is a well-known chemical compound that is widely used in sculpting materials and gauze bandages. While we have seen numerous applications of this substance in our daily lives, Plaster of Paris is a white powdered chemical compound that is hydrated calcium sulphate that is typically
8 min read
Biological Importance of Alkali and Alkaline Earth MetalsElements are arranged in a periodic table row-wise and column-wise according to similarities in their chemical and physical properties. The elements in the first column are known as Group 1 elements which have the following elements lithium, sodium, potassium, rubidium, Caesium and Francium. All the
10 min read